
R&D Innovation
R&D INNOVATION
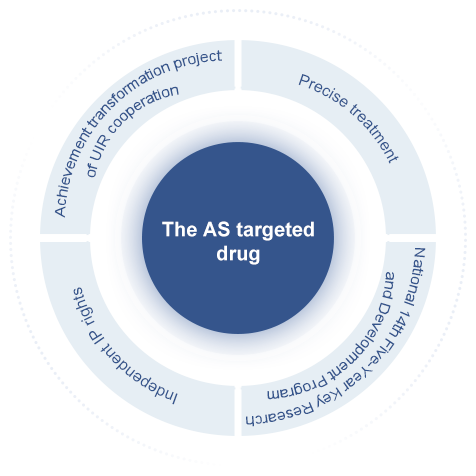
In a groundbreaking achievement, Inno Medicine has developed an innovative targeted therapy of atherosclerosis, resulting from a successful technology transfer collaboration with Beijing Anzhen Hospital. This remarkable advancement enables precise and effective treatment of atherosclerosis by employing a unique active targeting delivery system, ensuring the drug reaches the intended site to deliver its therapeutic benefits. YN001 has received IND approval from the US FDA and China NMPA, becoming the world's first cardiovascular targeted drug to enter clinical trials, is expected to become the first cardiovascular drug in history to obtain FDA approval through Accelerated Approval, and become a disruptive treatment method in this field. The project has been granted by the National 14th Five-Year Key Research and Development Program of China, and has obtained funding support from multiple research projects from the National Natural Science Foundation of China and the Beijing Natural Science Foundation.
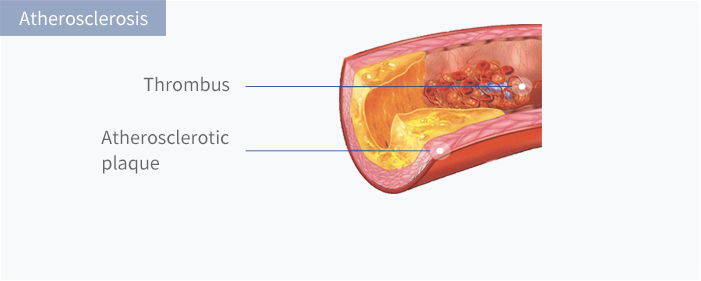
Atherosclerosis (AS) refers to the formation of atherosclerotic plaques in arteries which cause luminal stenosis. Once the plaque ruptures and forms thrombus, ischemic necrosis of the corresponding organs will occur, usually leading to irreversible damage
coronary atherosclerosis disease (coronary heart disease), cerebral atherosclerosis disease, peripheral atherosclerosis disease

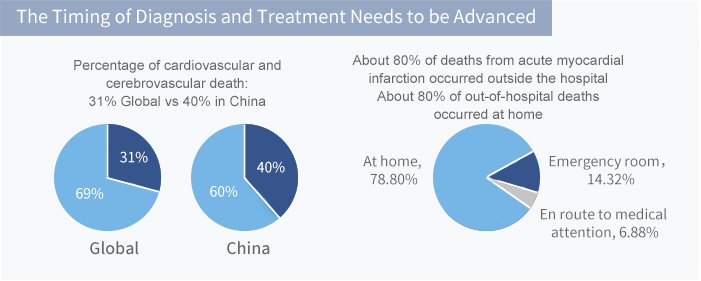
No Substantial and Clinical Effective Combination Protocol of Diagnosis and Treatment for AS can be Achieved with Current Methods
Huge Unmet Clinical Need and Market Space with Less Competition

Combination Protocol of Targeted Diagnosis and Treatment for Inflammatory Plaque Reversal by INNO
Beijing Inno Medicine Co., Ltd.
Address: Building 9, Area C, Xishan Creative Park, Haidian District, Beijing
Telephone number: +86 8259 9080
Copyright © Copyright 2020 Beijing Inno Medicine Co., Ltd. Beijing ICP Preparation No. 2020036980 京ICP备2020036980号